Abstract
Background: Severe Aplastic Anemia (SAA) is a rare bone marrow disorder characterized by inadequate levels of peripheral, multi-lineage blood cells.
Of the two available first-line treatments for SAA, allogeneic hematopoietic stem cell transplantation is limited by patient eligibility and donor availability, and immunosuppressive therapy (IST) is characterized by a significant proportion of non-responders, toxicity, and risk of transformation to diseases such as acute myelogenous leukemia. Patients who do not respond to treatments become transfusion dependent, which has a significant impact on patients' quality of life as well as healthcare costs.
Eltrombopag is the only TPO-R agonist approved for the treatment of refractory SAA. In a Phase I/II clinical trial, eltrombopag, given in association with IST, showed efficacy in patients with naive-SAA, and offers a significantly improved first-line alternative to patients affected by SAA (Townsley, et al 2017). In the US healthcare environment, there is a need to compare costs and consequences to understand value.
Objective: Evaluate eltrombopag as a first-line treatment versus IST alone for SAA from the American private healthcare system perspective.
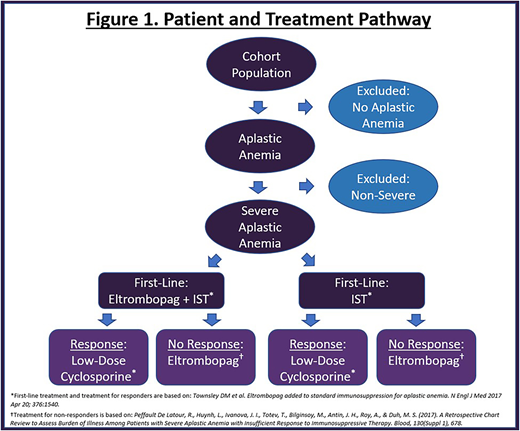
Methods: A responder model for newly diagnosed SAA patients was created to assess the treatment pathway and economic impact of including eltrombopag in addition to IST (antithymocyte globulin and cyclosporine A) as a first-line treatment. A simulated cohort with two treatment arms underwent 6 months of treatment with either eltrombopag in addition to IST or with IST alone and were followed for a 3-year time period. Each arm received a diagnostic test measuring response at 6 months. Patients who achieved complete or partial response in either arm received low-dose cyclosporine A as maintenance therapy for an additional 6 months of treatment. Patients who did not respond in either arm continued with eltrombopag monotherapy as a second-line therapy for an additional 6 months.
First-line therapy (eltrombopag with IST, IST alone), maintenance therapy (low-dose cyclosporine A), second-line therapy (eltrombopag monotherapy), administration, routine care, mortality and adverse event costs were included in the analysis. Workplace productivity related costs were not considered. Response rates, mortality, dosing, treatment duration and adverse event rates for each arm were based on a phase I/II trial (Townsley, et al 2017). Drug costs were obtained from a large online database (REDBOOK Online). Administration costs were based on the 2017 CMS Medical Fee Schedule. Routine care rates (visits, hospitalizations, tests and transfusions) were based on published data (Peffault De Latour, et al, 2017). Routine care, mortality and adverse event costs were based on CPT codes from the American Medical Association, HCUPnet and published data (Toner, et al 2011). All cost data are reported in 2018 US dollars. See figure 1 for details.
Results: In a simulated cohort with a population of one million, the annual incidence of aplastic anemia was 0.000234% and SAA accounted for 83.8% of those cases. The two treatment paths were compared for their consequences. Based on the clinical trial data, in the treatment arm with eltrombopag and IST, 94% of patients experienced treatment response relative to the IST arm where only 66% of patients experienced treatment response. Further, in the treatment arm with eltrombopag and IST, the patients experienced a reduced annual risk of mortality by 0.3% relative to the IST arm. Use of eltrombopag therapy as a first-line therapy produced a cost increase of $77,442 over 3 years. First-line drug costs accounted for an increase of $109,147, while improvements in response rates led to cost offsets for second-line drugs and produced $29,663 in savings. Adverse event, routine care and mortality costs had relatively negligible effects on either treatment arm over a 3-year time period. Sensitivity analyses confirmed the robustness of the analysis.
Conclusion: When following treatment approaches specified in clinical studies, high response rates combined with reduced risk of mortality and less usage of rescue medication, and a low disease incidence are likely to lead to manageable economic consequences with eltrombopag + IST therapy from the American private healthcare system perspective. In a simulated cohort with a population of one million, this was estimated to be $77,442 over 3 years.
Said:Novartis: Employment. Cai:Novartis: Employment. Forsythe:Novartis: Consultancy. Roy:Novartis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal